PhD Thesis Defense by Cüneyt Karakaya
Mesoporous Materials for Electrochemical Conversion
Time: September 1, 2021, 10:00 a.m.
Place: This thesis defense will be held online. You can join the presentation through the below link at the mentioned date and time
Join Zoom Meeting
https://kocun.zoom.us/j/91880412892
Meeting ID: 918 8041 2892
Passcode: 683108
Thesis Committee Members:
Assoc. Prof. Dr. Sarp Kaya (Advisor, Koç University)
Prof. Dr. Havva Funda Acar Yağcı (Koç University)
Assoc. Dr. Oktay Demircan (Boğaziçi University)
Prof. Dr. Can Erkey (Koç University)
Prof. Dr. Servet Turan (Eskişehir Technical University)
Abstract:
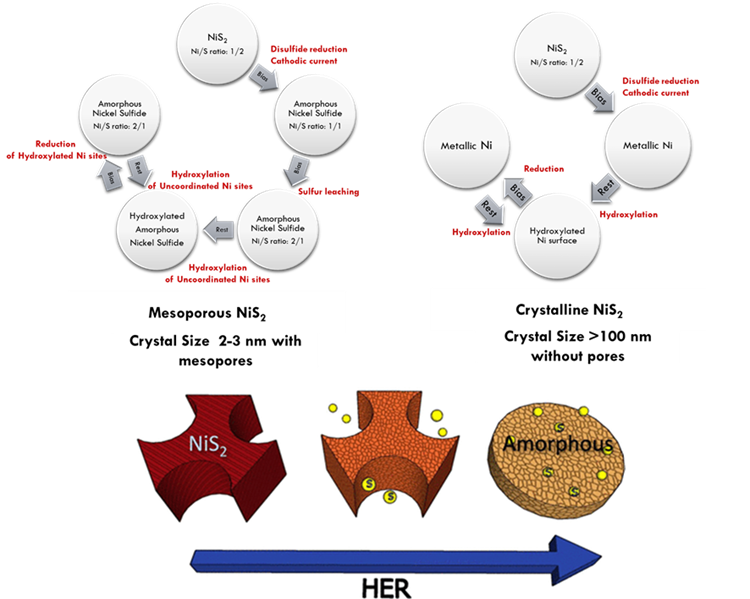
Cheap, abundant, and easily manufactured electrocatalysts with high efficiency and stability are required for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) to make renewable hydrogen production widespread. Transition metal chalcogenides and oxides have shown to be promising and versatile materials for catalyzing HER and OER in recent decades. Specifically, transition metal sulfides such as NiS2 and MoS2 offer great potential, their defective surface and edge site compositions, conductivities, and surface areas impose a significant effect on their electrochemical activities towards HER. On the other hand, mixed iron-nickel oxide based electrocatalysts have been intensively studied for OER. Engineering of abundant metal oxide/sulfide nanoparticles with a porous network by tuning the composition, structure, and morphology is highly promising to explore the most active and stable electrocatalyst. For this purpose, in this study, we developed a soft templating method to produce mesoporous nickel sulfides, mixed iron-nickel oxides, and molybdenum sulfide/oxide thin films. All synthesized materials were investigated by advanced characterization tools to identify their compositions, morphologies, and structures. Our soft templating method enables the production of mesoporous NiS2, Ni3S2, NiS, Ni7S6, and mixed compositions by altering the starting precursor compositions. The performances of nickel sulfides were tested towards HER in both acidic and alkaline electrolytes. Electrochemical tests with tracking the samples by ex-situ advanced material characterization tools reveal that mesoporous nickel sulfides act as pre-catalyst that transforms into superior active sulfur deficient nickel sulfide with the collapse of the mesoporous structure under HER in an alkaline electrolyte. A similar observation was observed in electrochemical tests of mesoporous NiS2 for OER. NiS2 was converted to Ni(OH)2 under a positive bias during OER. We synthesized the mesoporous NiFeOx, NiFe2O4, and Fe2O3 thin films with tunable compositions by changing the Ni/Fe precursors for OER in alkaline electrolytes. In the analysis of intrinsic activities, a peak in oxygen evolution activity was observed below %5 Fe content, leading to the formation of NiFeOx, where the Fe content decreases OER activity. The formation of NiFe2O4 slightly decreased OER activity, but mixed metal oxide was substantially more active than the pure NiO or Fe2O3. Fe impurities in KOH electrolyte electrochemical deposit on the electrocatalyst surface and enhance the OER activity of Ni-based electrocatalyst. With a cautious comparison of electrochemical tests results, Fe atoms in NiO lattice were found to boost OER activity substantially more compared to Fe deposition Ni-based metal oxide electrocatalyst. We also applied the soft templating strategy with minor modifications to produce mesoporous MoS3-MoS2-MoO3 thin films for HER in acidic media. With ex-situ analysis of structure and activity, MoS3 in the mesoporous framework was electrochemically reduced to more active MoS2. Overall, the soft templating strategy is a facile and scalable fabrication method to develop a cheap and efficient electrocatalyst with an integrated film structure. In addition, the mesoporous structure of electrocatalysts provides insights into understanding structure-activity relation, changes in the surface composition or morphology, and durability of electrocatalysts owing to the high surface area with ultra-small crystal sizes.
