PhD Thesis Defense by Samira Fatma Kurtğlu Öztülüm
Structural Factors Controlling the Stability of Atomically Dispersed Supported Iridium Catalysts
Time: September 21, 2021, 6:00 pm
Place: This thesis defense will be held online. You can join the presentation through the below link at the mentioned date and time
Join Zoom Meeting
https://kocun.zoom.us/j/92237928228
Meeting ID: 922 3792 8228
Passcode: 719160
Thesis Committee Members:
Assoc. Prof. Alper Uzun (Advisor, Koç University)
Prof. Dr. Can Erkey (Koç University)
Assoc. Prof. Uğur Ünal (Koç University)
Prof. Dr. Ramazan Yıldırım (Boğaziçi University)
Dr. Simon R. Bare (Stanford University)
Abstract:
Atomically dispersed supported metal catalysts provide many advantages such as, offering maximum utilization of noble metals, providing new and unprecedented catalytic properties, and obtaining structure-performance relationships thanks to their high degree of uniformity. Besides these advantages, they face several challenges which limits their utilization in industrially viable applications. This dissertation focuses on overcoming two of these challenges: their limited stability under reaction conditions and their limited metal loadings.
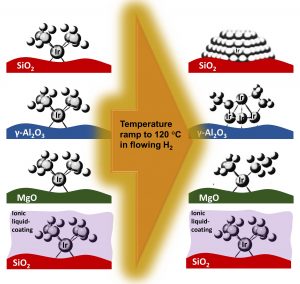
SiO2, g-Al2O3, and MgO-supported Ir(C2H4)2 complexes at 1 wt% Ir loading were synthesized to evaluate them for their stability under a pure H2 flow during a ramp from room temperature to 120 °C by in-situ X-ray absorption spectroscopy (XAS) measurements. Fourier transform infrared spectroscopy (FTIR) measurements and density functional theory calculations confirmed the strong, intermediate, and weak electron donor tendency of MgO, g-Al2O3, and SiO2, respectively, to the Ir centers. Extended X-ray absorption fine structure (EXAFS) indicated nanoparticle formation on the weak-electron-donor SiO2 and small Ir4 cluster formation on the intermediate-electron-donor g-Al2O3, upon H2 treatment. The Ir complexes remained intact when supported on the strong-electron-donor MgO under identical treatment conditions. When the most severely aggregating sample, Ir(C2H4)2/SiO2, was coated with an electron-donor ionic liquid (IL), 1-n-ethyl-3-methylimidazolium acetate ([EMIM][OAc]), aggregation could be hindered, as well. Results illustrate that the electron density on Ir controls their aggregation behavior.
A special support, reduced graphene aerogel (rGA), was used to support Ir(C2H4)2 complexes at a high loading of 9.9 wt%. FTIR and EXAFS data of the fresh sample evidenced the site-isolated Ir centers in this catalyst. Next, under a flow of equimolar ethylene and H2 during a ramp from room temperature to 100 °C and a subsequent 30 min isothermal period at 100 °C, small Ir4 clusters were formed on rGA. When the feed condition was changed to a H2-rich feed flow (H2:C2H4 = 2) and then a pure H2 flow for 30 min each at 100 °C, Ir4 clusters were transformed into small Ir6 clusters, confirmed by in-situ XAS and scanning transmission electron microscopy. These small clusters still offer atomic dispersion of Ir, by providing advantages for ethylene hydrogenation thanks to the neighboring Ir sites, easing H2 activation. It was found that, Ir clusters provide higher catalytic performance compared to their site-isolated analogues in the following order: Ir/rGA << Ir4/rGA < Ir6/rGA. The unique properties of rGA lead to the stabilization of small Ir6 clusters at a significantly high metal loading.
Next, rGA-supported Ir(C2H4)2 complexes at a loading of 9.9 wt% were coated with an electron donating IL ([EMIM][OAc]) aiming to stabilize atomically dispersed Ir complexes during reaction. In-situ EXAFS data confirmed that subjecting the [EMIM][OAc]-coated catalyst to ethylene hydrogenation conditions under varying H2:C2H4 ratios at 100 °C, followed by a pure H2 treatment at 100 °C did not result in any aggregation. COSMO-RS calculations point out that besides the electronic effect, a filtering effect of the IL takes place. IL coatings offer a broad potential for the stabilization of atomically dispersed metal catalysts at such a high surface metal density.
rGA was further used as a support for Ir with reactive ethylene ligands, to reach an exceptionally high metal loading of 23.8 wt%. In-situ XAS data confirmed that the catalyst remained stable under working state for 2 h under ethylene hydrogenation with equimolar ethylene and H2 flow. Increasing the temperature at the aforementioned feed conditions to 100 °C, retaining the catalyst for 30 min at equimolar flow, 40 min at H2-rich feed flow (H2:C2H4 = 2) and finally 40 min at pure H2 flow lead to the formation of Ir nanoclusters (~ 1 nm) characterized by Ir–Ir first and second shell coordination numbers of 6.6 ± 0.3 and 2.6 ± 0.9, respectively.
Another aim was to assess the thermal stability limits of ILs on metal oxides. 29 different ILs were immobilized on MgO and SiO2, to represent basic and acidic supports, respectively. The maximum tolerable temperatures of bulk ILs, and when they were immobilized on the metal oxides were evaluated by thermogravimetric analysis. To investigate the factors affecting the thermal stability of ILs on metal oxides, changing alkyl chain length, the methylation on C2 site in imidazolium ILs, the change in substituent position in the ring of pyridinium ILs, the change in the anion type, and the change in the IL family (imidazolium, pyridinium, piperidinium, pyrrolidinium) was investigated. Results show that the basicity of the support and the hydrophilicity of the anion and their resulting interactions is key in determining the thermal stability limits of ILs on metal oxides. Findings present the chance to pick a suitable IL/metal oxide pair for their use in catalytic systems, such as their utilization in stabilizing atomically dispersed supported metal catalysts.
Results presented in this dissertation provide the factors determining the stability of atomically dispersed iridium complexes on supports and the use of rGA as a promising support material to stabilize Ir complexes during reaction and to reach a high metal loading. Data presented show the opportunities of using IL coatings to hinder aggregation of these catalysts, even under harsh conditions, such as pure H2 at elevated temperatures.
